Mesenchymal stem cells: precursor hierarchy
Irina Shipounova (Nifontova), Daria Svinareva, Josef Chertkov, Nina Drize
Accepted 21 November 2008
Published 15 December 2008
Summary
The hierarchy of stromal precursors is the focus of this research. It has been previously shown that transplantation of the bone marrow plug under the renal capsule of the syngeneic animal leads to the formation of the foci of ectopic hematopoiesis, where a stromal microenvironment is formed by the donor's mesenchymal stem cells (MSC). In the irradiated recipients such foci are 2-3 times larger than in non-irradiated foci due to "inducible" precursors that are more differentiated than MSC. Along with the in vivo tests, the method of in vitro estimation of concentration of clonogenic stromal precursors (CFU-F) is widely used. However, the hierarchical arrangement of the described precursors is still unclear. This study describes the alterations in the number of mentioned precursors in the ectopic hematopoietic foci formed in the irradiated recipients. CFU-F was shown to be the closest MSC progeny thus far, while "inducible" precursor cells – stromal multipotent precursors – are at a lower position in the hierarchy and possibly enlarge the hematopoietic territory in the irradiated recipients directly.
Keywords
Hematopoietic ectopic foci, long-term bone marrow culture, hierarchy of precursor cells, mesenchymal stem cells, colony forming unit-fibroblast
Introduction
Stromal cells of the hematopoietic microenvironment are the progeny of mesenchymal stem cells (MSC). MSC are non-hematopoietic multipotent stem cells able to differentiate into different cells lines, such as osteoblasts, adipocytes, chondrocytes, fibroblasts, and other cell lines [1]. The ability to self-renew has not been proven for human MSC [2]; however, these cells are known to have high proliferative potential when cultured. Murine MSC are able to transfer a hematopoietic microenvironment in vivo at least 9 times, confirming their ability for self-maintenance [3]. Until now, the data about phenotypical markers of MSC has not been developed sufficiently [4], but MSC are able to express the number of non-specific markers [5]. It was shown recently that fibroblast activation protein perfectly identifies mesenchymal stromal cells [6]. The compartment of stromal precursor cells can be characterized by physiological methods according to the first 25 years of hematopoietic stem cells (HSC) research. Explantation of bone marrow cell suspension into culture flasks led to the development of discrete fibroblast-like colonies. Each colony represents a clone produced by single clonogenic precursor cells–colony-forming unit fibroblasts (CFU-F) [7]. CFU-F are of mesenchymal origin and do not develop from HSC [8, 9]. CFU-F are heterogenic cell populations and some of them possess high proliferative potential; their ability to differentiate could be associated with MSC [10]. The transplantation of the pull of colonies into the organism leads to the development of different tissues, including bone and adipose tissues [11, 12]. The comparison of CFU-F with MSC is questionable because CFU-F are able to differentiate inside the diffusion chambers after implantation to the organism, but it is not known whether they are able to transfer the full microenvironment or self-maintain. Recent data suggests that CD146+ CFU-F with high proliferative capacity, are able to transfer the microenvironment, but this can be applied only to rare cells in the bone marrow [13]. Moreover, human multipotent stromal cells readily form single-cell-derived colonies, which are heterogeneous because cells from a colony form new colonies that vary in size and differentiation potential [14]. Several growth factors influence the CFU-F growth, and four of them are necessary for CFU-F development: PDGF, bFGF, TGFβ and EGF [15]. The proliferative potential of CFU-F is not studied in detail, but it is known that most of the CFU-F in the organism are not cycling and remain in the “Go” phase [16]. However, CFU-F start to proliferate when transferred to cultures.
The compartments of MSC and HSC have hierarchical structures. This is well known for HSC, but in the case of MSC it was discovered for the first time in experiments with ectopic foci formation in vivo. At 6 weeks after implantation of the donor bone marrow plug under the renal capsule of the syngeneic recipient, ectopic hematopoietic foci developed at the site of transplantation. In such foci, stromal cells belong to the donor, and hematopoietic cells belong to the recipient. Multiple histological examinations during foci formation have revealed that the hematopoietic microenvironment was built de novo. The method of ectopic foci formation is not only qualitative, but also quantitative. The size of the hematopoietic territory is defined by the number of MSCs transplanted, and restricted accordingly to the number of niches transferred with the bone marrow plug; since the foci size is proportional to the amount of implanted bone marrow. It is possible to transfer the foci under the renal capsule of the secondary recipient. In this case foci again would be built de novo due to the presence of MSC. The foci sustain at least 9 re-transplantations that prove the high capability of MSC to self-renew [3]. The radio-sensitivity of MSC is much lower than that of HSC [17]. In summarizing this data it is possible to conclude that stromal precursor cells that are able to transfer the microenvironment are true stem cells and the method of ectopic foci formation allows the opportunity to estimate their numbers in bone marrow.
In irradiated recipients foci formed after the implantation of bone marrow plugs are enlarged 2-3 fold in comparison with foci developed in non-irradiated mice. However, the re-transplantation of these enlarged foci to non-irradiated recipients led to the formation of foci of normal size. Thus, in the enlarged foci the number of MSC do not increase, and the microenvironment created in the irradiated recipients is enlarged by cells other than MSC inducible precursor cells, which are not able to self-renew [18]. Such stromal multipotent precursor (SMP) cells have the position in the hierarchy of MSC similar to the position of the multipotent precursor in the hierarchy of HSC. The position of CFU-F in the hierarchy of MSC is unknown. It is possible to define it by studying the concentration of CFU-F in ectopic foci formed in non-irradiated and irradiated recipients. There are three possible variants for the irradiated recipients: the concentration of CFU-F could increase, decrease, or remain unchanged. We suppose that CFU-F could be considered to be the progeny of SMP in cases where their concentration in enlarged foci do not change and their numbers increase 2-3 fold according to the foci size. In cases of both decreased concentration and number of CFU-F in enlarged foci, it is possible to assume that these precursor cells with limited proliferative potential are used irreversibly to form SMP, thus CFU-F are located higher than SMP in the hierarchy of MSC and have the position directly before SMP. If the actual number of CFU-F in the enlarged foci do not change, one could conclude that there are precursors that do not take part in the formation of the microenvironment and represent special populations of stromal clonogenic cells.
The measurement of the concentration and number of CFU-F in ectopic foci in irradiated and non-irradiated recipients performed in this study defines the position of CFU-F immediately after MSC and before SMP in the hierarchy of mesenchymal stem cells.
Materials and methods
The hybrid mice (СВАхC57Bl/6) F1 at the age of 12-16 weeks at the beginning of the experiment were used. The animals were irradiated on the IPK 137Cs irradiator with a dose rate of 16 сGy/min.
For CFU-F analysis 106 bone marrow cells were seeded into the T25 flask in 5 ml αМЕМ (ICN) with 20% fetal bovine serum (Hyclone) and 5 ng/ml basic Fibroblast Growth Factor (bFGF) (donated by Gasparian M.E., Lab. of Protein engineering, IBC, RAS). Cells were cultivated for 14 days in 370С and 5% СО2. Formed colonies were stained with 0.1% crystal violet on 20% methanol and counted under the inverted microscope (the colony was counted if it contained no less than 50 cells).
For cloning of individual CFU-F, bone marrow cells were planted into a 96-well plate in concentrations from 30,000 to 50,000 of nucleated cells per well in standard media. In this cultivation system the most convenient concentration turned out to be 30,000 cells per well (Table 1). In this assay the frequency of CFU-F was calculated by means of the Poisson equation:
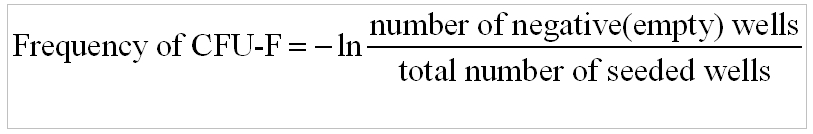
Table 1. Frequency of CFU-F in the bone marrow of mice

Clones from the wells containing single colonies were transferred into 24-well plates, then into 6-well plates, and finally into T25 flasks. For each procedure, the cells were washed with Versen solution and detached with 0.25% tripsin solution. The proliferative potential of CFU-F was estimated by their ability to form a confluent monolayer during sequential transfers from small to large available growth areas. The number of divisions performed by CFU-F progeny was evaluated by the assumption that the number of cells in the confluent monolayer increases proportionally to the growth area. For instance, the bottom square of the well in a 96-well plate is 0.32 cm2; 24-well, 1.88 cm2; 6-well, 9.4 cm2; and a Т25 flask is 25 cm2. If the confluent monolayer is transferred from one well of a 96-well plate into one well of a 24-well plate, the size of available growth area increases 6-fold; from a 24-well plate to a 6-well plate is 5-fold; and from a 6-well plate into a T25 flask is 2.3-fold. One could calculate easily that in order to cover the bottom surface of one well of a 6-well plate the cells should be divided 5 times (counting from confluent monolayer of one well of a 96-well plate) and have to go through one more mitosis to reach the confluent monolayer in flask T25.
The method of ectopic foci formation has been described previously [18]. In brief, the bone marrow plug was implanted under the renal capsule of non-irradited or irradiated with the dose of 6-10 Gy syngeneic recipient mice. Hematopoiesis in animals irradiated with 10 Gy was carried out by the intravenous injection of syngeneic bone marrow cells (no less than 106 bone marrow cells per mouse). Six weeks later, the size of the developed ectopic foci was measured by the calculation of the number of nucleated cells inside the foci. The number of CFU-F in ectopic foci was measured by the standard method described above.
Statistical analysis was performed using Student's t-test.
Results and discussion
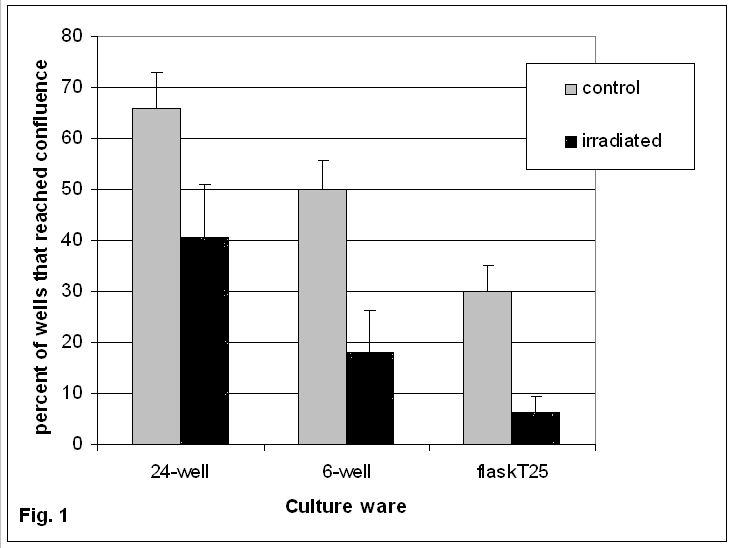
CFU-F is a heterogenic group of stromal precursor cells with different proliferative potential. The concentration of CFU-F per 106 bone marrow cells in non-irradiated and irradiated with 6 Gy mice 1.5 to 3 months before the analysis does not differ significantly (68.4 ± 8.3 versus 80.6 ± 7.4 correspondingly). CFU-F derived colonies from the bone marrow of non-irradiated and sub-lethally irradiated mice after cloning (seeding concentration 30,000 cells per well of the 96-well plate) were sequentially re-transplanted to 24- and 6- well plates, and subsequently to a T25 flask (Fig. 1). Cells from only 30% of CFU-F derived colonies from non-irradiated mice and 6.25% from irradiated ones were able to undergo more than six rounds of mitosis. After irradiation, the proliferative potential of CFU-F decreased while their concentration in the bone marrow did not change. Precursor cells that survived the irradiation filled the concentration of CFU-F in the bone marrow that resulted in the exhaustion of precursors with high enough proliferative potential. On the contrary, after treatment of mice with different cytostatic agents the concentration of CFU-F in the bone marrow decreased dramatically, and even at 6 weeks after the end of treatment the concentration was not restored [19]. Taking into consideration that stromal cells are highly radio-resistant, one could suggest that CFU-F regenerate after irradiation much more efficiently than after cytostatics.
Figure 1. Proportion of CFU-F derived colonies from bone marrow of non-irradiated and irradiated mice which are able to grow to the confluent monolayer in different culture ware.
Data are shown as the means (±SEM).
Axis of abscissa: culture ware
Axis of ordinate: percent of wells that reached confluence
The implantation of confluent monolayers from 9 CFU-F derived colonies, grown in T25 flasks after sequential re-transplantations under the renal capsule of syngeneic recipients, resulted in no foci formation. Therefore, CFU-F derived cells are not able to transfer the hematopoietic microenvironment. Probably only the very rare CD146 positive CFU-F are able to proliferate and differentiate in vivo forming bone marrow hematopoietic microenvironment [13].
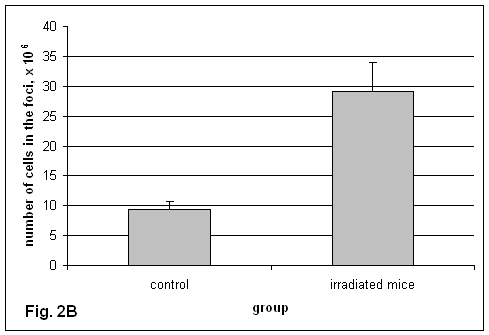
CFU-F in ectopic foci has not been characterized yet. The concentration of CFU-F in the ectopic foci turned out to be lower than in the bone marrow (Fig. 2А). The concentration of CFU-F in ectopic foci formed in irradiated recipients was reduced 20-fold in comparison with foci formed in non-irradiated recipients. As the size of the foci in irradiated recipients is enlarged significantly (Fig. 2B), the actual number of CFU-F in such foci is only 3-fold lower than in foci formed in non-irradiated recipients (Fig. 2C).
Figure 2. CFU-F in the ectopic foci formed in non-irradiated and irradiated recipients.
А. Concentration of CFU-F in the ectopic foci and bone marrow of non-irradiated mice.om bone marrow of non-irradiated and irradiated mice which are able to grow to the confluent monolayer in different culture ware.
Data are shown as the means (±SEM).
Axis of abscissa: group
Axis of ordinate: number of CFU-F per 106 cells
B. The size of ectopic foci formed in non-irradiated and irradiated recipients.
Data are shown as the means (±SEM).
Axis of abscissa: group
Axis of ordinate: number of nucleated cells in the foci, х 106

C. CFU-F number in the ectopic foci.
Data are shown as the means (±SEM).
Axis of abscissa: group
Axis of ordinate: number of CFU-F per foci
Thus, it is possible to suggest that the position of CFU-F in the hierarchy of MSC is higher than the position of SMP, but lower than MSC.
Stromal growth factor produced by bones of irradiated recipients induces the formation of enlarged foci and has been shown to persist in blood [20]. Addition of murine sera to CFU-F cultivation media increased their number [21]. However, addition of 2.5% of sera from irradiated mice to cultivation media for CFU-F decreased their number dramatically (Fig. 3). A high number of separate cells that were not producing clones could be seen on the flask bottom, which is atypical for the method and the mode of CFU-F growth. One could suggest several causes for the decreasing CFU-F number in the presence of sera from irradiated mice. It is impossible to neglect the difficulties in CFU-F against the background of a multitude of separate cells revealed after the addition of sera from irradiated mice. On the other hand, stromal growth factor from this serum obviously stimulated the differentiation of CFU-F progeny, as if inducing hematopoietic territory as it happens in vivo during the development of hematopoietic ectopic foci in irradiated recipients. The in vitro decrease of CFU-F concentration in this case is also analogous to the results obtained in vivo. Therefore, the data concerning the effect of the addition of sera from irradiated mice on CFU-F growth also supports the proposed positions of SMP and CFU-F in the hierarchy of MSC.
Figure 3. Effect of sera from non-irradiated and irradiated mice on the number of CFU-F.
Data are shown as the means (±SEM).
Axis of abscissa: group
Axis of ordinate: number of CFU-F per 106 cell
Analysis of the total data positioned CFU-F in the hierarchy of stromal precursor cells (Fig. 4). These heterogenic groups are not able to self-renew, but their high proliferative potential is the result of being the progeny of MSC. SMP, stimulated by stromal growth factor, enlarges the hematopoietic territory in irradiated recipients and takes place at a lower position than CFU-F. There is still a lot of research that needs to be done regarding the hierarchy of MSC.
Figure 4. Hierarchy of MSC

Acknowledgements
This study was supported by Grant 07-04-00183-а of Russian Fund of Fundamental science.
References
1. Caplan AI. The mesengenic process. Clin Plast Surg. 1994;21:429-435.
2. Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, and Keating A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393-395.
3. Chertkov JL and Gurevitch OA. Self-maintenance ability and kinetics of haemopoietic stroma precursors. Cell Tissue Kinet. 1980;13:535-541.
4. Short B, Brouard N, Occhiodoro-Scott T, Ramakrishnan A, and Simmons PJ. Mesenchymal stem cells. Arch Med Res. 2003;34:565-571.
5. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, and Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317.
6. Bae S, Park CW, Son HK, Ju HK, Paik D, Jeon CJ, Koh GY, Kim J, and Kim H. Fibroblast activation protein alpha identifies mesenchymal stromal cells from human bone marrow. Br J Haematol. 2008;142:827-830.
7. Friedenstein AJ, Chailakhjan RK, and Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393-403.
8. Castro-Malaspina H, Gay RE, Jhanwar SC, Hamilton JA, Chiarieri DR, Meyers PA, Gay S, and Moore MA. Characteristics of bone marrow fibroblast colony-forming cells (CFU-F) and their progeny in patients with myeloproliferative disorders. Blood. 1982;59:1046-1054.
9. Koide Y, Morikawa S, Mabuchi Y, Muguruma Y, Hiratsu E, Hasegawa K, Kobayashi M, Ando K, Kinjo K, Okano H, and Matsuzaki Y. Two distinct stem cell lineages in murine bone marrow. Stem Cells. 2007;25:1213-1221.
10. Owen M and Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42-60.
11. Friedenstein AJ, Chailakhyan RK, and Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20:263-272.
12. Bennett JH, Joyner CJ, Triffitt JT, and Owen ME. Adipocytic cells cultured from marrow have osteogenic potential. J Cell Sci. 1991;99(Pt 1): 131-139.
13. Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, and Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324-336.
14. Ylostalo J, Bazhanov N, and Prockop DJ. Reversible commitment to differentiation by human multipotent stromal cells in single-cell-derived colonies. Exp Hematol. 2008.
15. Kuznetsov SA, Friedenstein AJ, and Robey PG. Factors required for bone marrow stromal fibroblast colony formation in vitro. Br J Haematol. 1997;97:561-570.
16. Kaneko S, Motomura S, and Ibayashi H. Differentiation of human bone marrow-derived fibroblastoid colony forming cells (CFU-F) and their roles in haemopoiesis in vitro. Br J Haematol. 1982;51:217-225.
17. Chertkov JL and Gurevitch OA. Radiosencitivity of precursors of hematopoietic microenvironment. Radiat Res. 1979;79:177-186.
18. Chertkov JL and Gurevitch OA. Hematopoietic stem cell and its microenvironment. Moscow: Meditzina; 1984.
19. Nifontova I, Svinareva D, Petrova T, and Drize N. Sensitivity of Mesenchymal Stem Cells and Their Progeny to Medicines Used for the Treatment of Hematoproliferative Diseases. Acta Haematol. 2008;119:98-103.
20. Drize NI, Ershler MA, and Chertkov IL. Radiation-induced hemopoietic cell growth factor: detection in a culture. Bull Exp Biol Med. 2001;132:1213-1215.
21. Abe R, Donnelly SC, Peng T, Bucala R, and Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556-7562.
Accepted 21 November 2008
Published 15 December 2008